FRESH Bioprinting of Soft Biomaterials
FRESH Bioprinting of Soft Biomaterials
Franca Scocozza1, MSc, Milena Savioli Lopes2, PhD, Volodymyr Kuzmenko2, PhD, Itedale Namro Redwan2, PhD
1Dept. of Civil Engineering and Architecture, University of Pavia, Italy
2CELLINK, Gothenburg, Sweden
Abstract
In extrusion-based 3D bioprinting, working with soft materials, like collagen, presents a com- plex challenge given their inability to retain a de- sired shape following extrusion.
One solution is freeform reversible embedding of suspended hydrogels (FRESH), a method of biofabrication which provides a temporary support and prevents the collapse or deformation of a construct during bioprinting. LifeSupport™, a commonly used gel- atin-based support bath for FRESH bioprinting, offers the added advantage that it can be easily removed after printing. This study aims to optimize the FRESH bioprinting of several popular biomate-rials using LifeSupport bath to provide BIO X users with established printing protocols. To show the versatility of FRESH bioprinting, we chose three soft biomaterials with different gelation mechanisms (thermal self-assembly, photo, and ionic). All biomaterials were rheologically characterized, optimized for printing and, finally, bioprinted with cells.
Franca Scocozza1, MSc, Milena Savioli Lopes2, PhD, Volodymyr Kuzmenko2, PhD, Itedale Namro Redwan2, PhD
1Dept. of Civil Engineering and Architecture, University of Pavia, Italy
2CELLINK, Gothenburg, Sweden
Abstract
In extrusion-based 3D bioprinting, working with soft materials, like collagen, presents a com- plex challenge given their inability to retain a de- sired shape following extrusion.
One solution is freeform reversible embedding of suspended hydrogels (FRESH), a method of biofabrication which provides a temporary support and prevents the collapse or deformation of a construct during bioprinting. LifeSupport™, a commonly used gel- atin-based support bath for FRESH bioprinting, offers the added advantage that it can be easily removed after printing. This study aims to optimize the FRESH bioprinting of several popular biomate-rials using LifeSupport bath to provide BIO X users with established printing protocols. To show the versatility of FRESH bioprinting, we chose three soft biomaterials with different gelation mechanisms (thermal self-assembly, photo, and ionic). All biomaterials were rheologically characterized, optimized for printing and, finally, bioprinted with cells.
APPLICATION NOTE
Introduction
In the span of a few years, 3D bioprinting has become one of the most popular techniques for precisely placing biomaterials and cells in 3D space to biofabricate constructs that recreate the structure and function of complex biological systems, from cellular to organ scale (Shiwarski, 2021). Although many different technologies and bioprinting strategies have been proposed for bioprinting complex tissues with very high resolution, extrusion- based bioprinting remains the most popular thanks to its versatility, low cost, and ease of use.
Unfortunately, extrusion-based 3D bioprinting of complex structures is difficult with soft liquid- like biomaterials (eg,collagen or decellularized extracellular matrix) due to gravity and subsequent loss of print fidelity. To overcome this, researchers have developed photo-crosslinkable, temperature- sensitive, and rheologically modified biomaterials to counter the effects of gravity and enable 3D bioprinting in the air (Adib, 2020; Heid, 2020). However, these approaches often require compromises in terms of biological properties and stringent bioprinting parameters (Hazur, 2020).
Freeform reversible embedding of suspended hydrogels (FRESH)
method has been developed as a solution to this issue by providing a temporary support structure (referred to in the following text as a support bath) to prevent the collapse and deformation of the constructs during printing (Hinton, 2015). The support bath has Bingham plastic properties that make the support bath behave as a hard body under low shear stresses and as a viscous fluid under high shear stresses. In this way, the printhead nozzle can move throughout the bath during printing and extrude biomaterial in the empty space created (Wu, 2011). After FRESH bioprinting, the support bath is melted away and the construct released.
FRESH bioprinting allows using a variety of biomaterials with different gelation mechanisms that can be printed in the wide range of support baths available on the market (based on gelatin, alginate, Carbopol, agarose, gellan gum, hyaluronic acid, etc.). Among these, gelatin-based support bath is one of the most used for FRESH bioprinting thanks to biocompatibility, low cost, and thermoreversible properties that make it possible to print constructs at room temperature and melt the bath at physiological temperature after printing. This is a very gentle process that is compatible with cells and proteins, meaning that even extremely soft scaffolds can be printed successfully and then released without losing essential biological properties.
This study aims to optimize the 3D bioprinting of three different low viscosity soft biomaterials with different gelation mechanisms using the FRESH bioprinting method with LifeSupport, a sterile gelatin based support bath provided by AdvancedBioMatrix. (Lee, 2019; Jeon, 2019). The optimization process included rheological characterization, adjustment of printing parameters and a proof-of-concept cell study with established bioprinting protocols.
Materials and methods
PREPARATION OF MATERIALS
An established protocol was used for the
preparation of the LifeSupport bath (Advanced BioMatrix, 5244): i) add 40mL of cold (4°C) suspension media (eg, PBS, crosslinker solution, cell medium) to a 2 g of LifeSupport, ii) vortex and shake the solution, iii) let stand for 15 minutes at 4°C to allow LifeSupport to fully rehydrate, iv) centrifuge the rehydrated LifeSupport twice for 1.5 minutes at 1500 rpm, v) pour off the liquid supernatant, vi) settle the LifeSupport in the printing substrate (eg,Petri dish or well plate). For the detailed preparation protocol, one can refer to the LifeSupport webpage. For this study, we selected soft biomaterials with three different gelation mechanisms: collagen with thermal self-assembly, methacrylated collagen with photocrosslinking, and sodium alginate with ionic crosslinking. For printing with collagens, the suspension medium for the bath was PBS, and for printing with alginate, the suspension medium for the bath contained 0.1% (w/v) CaCl2.
TeloCol-10 (Advanced BioMatrix, 10 mg/mL, 5226) is a type I bovine collagen solution that can be thermally crosslinked at 37°C. To prepare 1 mL of 6 mg/mL TeloCol® solution for printing, 600 µL of 10 mg/mL stock solution was transferred into a sterile Eppendorf using a positive displacement pipette. 100 µL of 10X PBS was added and mixed. 7.0 μL of sterile 1M NaOH was used to neutralize the solution and achieve the physiologic pH of 7.2. 293 μL of deionized water was added to reach the final concentration of 6 mg/mL. For printing
Freeform reversible embedding of suspended hydrogels (FRESH)
method has been developed as a solution to this issue by providing a temporary support structure (referred to in the following text as a support bath) to prevent the collapse and deformation of the constructs during printing (Hinton, 2015). The support bath has Bingham plastic properties that make the support bath behave as a hard body under low shear stresses and as a viscous fluid under high shear stresses. In this way, the printhead nozzle can move throughout the bath during printing and extrude biomaterial in the empty space created (Wu, 2011). After FRESH bioprinting, the support bath is melted away and the construct released.
FRESH bioprinting allows using a variety of biomaterials with different gelation mechanisms that can be printed in the wide range of support baths available on the market (based on gelatin, alginate, Carbopol, agarose, gellan gum, hyaluronic acid, etc.). Among these, gelatin-based support bath is one of the most used for FRESH bioprinting thanks to biocompatibility, low cost, and thermoreversible properties that make it possible to print constructs at room temperature and melt the bath at physiological temperature after printing. This is a very gentle process that is compatible with cells and proteins, meaning that even extremely soft scaffolds can be printed successfully and then released without losing essential biological properties.
This study aims to optimize the 3D bioprinting of three different low viscosity soft biomaterials with different gelation mechanisms using the FRESH bioprinting method with LifeSupport, a sterile gelatin based support bath provided by AdvancedBioMatrix. (Lee, 2019; Jeon, 2019). The optimization process included rheological characterization, adjustment of printing parameters and a proof-of-concept cell study with established bioprinting protocols.
Materials and methods
PREPARATION OF MATERIALS
An established protocol was used for the
preparation of the LifeSupport bath (Advanced BioMatrix, 5244): i) add 40mL of cold (4°C) suspension media (eg, PBS, crosslinker solution, cell medium) to a 2 g of LifeSupport, ii) vortex and shake the solution, iii) let stand for 15 minutes at 4°C to allow LifeSupport to fully rehydrate, iv) centrifuge the rehydrated LifeSupport twice for 1.5 minutes at 1500 rpm, v) pour off the liquid supernatant, vi) settle the LifeSupport in the printing substrate (eg,Petri dish or well plate). For the detailed preparation protocol, one can refer to the LifeSupport webpage. For this study, we selected soft biomaterials with three different gelation mechanisms: collagen with thermal self-assembly, methacrylated collagen with photocrosslinking, and sodium alginate with ionic crosslinking. For printing with collagens, the suspension medium for the bath was PBS, and for printing with alginate, the suspension medium for the bath contained 0.1% (w/v) CaCl2.
TeloCol-10 (Advanced BioMatrix, 10 mg/mL, 5226) is a type I bovine collagen solution that can be thermally crosslinked at 37°C. To prepare 1 mL of 6 mg/mL TeloCol® solution for printing, 600 µL of 10 mg/mL stock solution was transferred into a sterile Eppendorf using a positive displacement pipette. 100 µL of 10X PBS was added and mixed. 7.0 μL of sterile 1M NaOH was used to neutralize the solution and achieve the physiologic pH of 7.2. 293 μL of deionized water was added to reach the final concentration of 6 mg/mL. For printing
APPLICATION NOTE
with cells, the deionized water was replaced with cell medium. For more details, access the FRESH Bioprinting Protocol: TeloCol-10.
with cells, the deionized water was replaced with cell medium. For more details, access the FRESH Bioprinting Protocol: TeloCol-10.
PhotoCol (Advanced BioMatrix, 5198) is a lyo- philized methacrylated type I collagen that can be thermally crosslinked at 37°C (mild crosslinking) and subsequently photocrosslinked for complete mechanical stabilization. First, the lyophilized PhotoCol® biomaterial was reconstituted using 20 mM acetic acid to a concentration of 10 mg/mL. To prepare 1 mL of 6 mg/mL PhotoCol solution for printing, 600 µL of 10 mg/mL stock solution was transferred into a sterile Eppendorf using a positive displacement pipette. The photoinitiator LAP (AdvancedBioMatrix, 5205) was dissolved in the neutralization solution to achieve a concetration of 0.25% (w/v) in the final bioink. To neutralize 600 µL of 10 mg/mL, 45 μL of neutralization solution/ LAP was used to reach the physiologic pH of 7.2. 355 μL of deionized water was added to reach the final concentration of 6 mg/mL. For printing with cells, the deionized water was replaced with cell medium. For more details, access the FRESH Bioprinting Protocol: PhotoCol.
Alginate Lyophilizate (CELLINK, VLB000000201)
provides a medium viscosity alginate solution sta- ble at room temperature and ionically crosslink- able with CaCl₂. Lyophilized alginate was reconsti- tuted to 2% (w/v) using Reconstitution Agent M (CELLINK, IK200000050). For cell printing, algi- nate solution was mixed with cell suspension in cell medium with a volume ratio of 9:1. For more details, access the FRESH Bioprinting Protocol: Alginate.
RHEOLOGY
Rheological measurements of LifeSupport and collagen biomaterials were performed using an oscillatory shear mode on a rotational Discovery HR-10 Rheometer (TA Instruments) with an aluminum 20mm parallel plate setup with a 0.5mm gap. Temperature ramps were evaluated from5°C to 37°C for collagen materials and from 10°C to 37°C for LifeSupport bath, with a ramp rate of 1°C/min, constant shear strain of 0.5%, and frequency of 1 Hz for all tests. The final stiffness of both collagen materials was measured under constant shear strain and frequency of 0.5% and 1 Hz, respectively, after loading the samples into a 5°C plate and instantaneously increasing the temperature to 37°C for thermal crosslinking. For PhotoCol, an additional step was introduced by exposing the sample to photocrosslinking for 30seconds, using an external LED lamp with near-UV light wavelength (405 nm).
OPTIMIZATION OF FRESH BIOPRINTING
PROCESS
FRESH bioprinting with LifeSupport involves dif- ferent steps shown in Figure 1. The process starts with adding a suspension medium (water, PBS, crosslinking solution, etc.) to the gelatin powder (Figure 1A). The solution is mixed and centrifuged to form the LifeSupport bath (Figure 1B), which is used to fill up a well plate (Figure 1C) or Petri dish. After printing (Figure 1D), the well plate is placed into an incubator at 37°C for 30 minutes for LifeSupport melting (Figure 1E) and the release of printed construct (Figure 1F).
To optimize FRESH bioprinting, one must select an appropriate needle size and adjust printing parameters such as pressure and speed. In our study, the printing optimization was performed using both 22 G and 25 G 1-inch length needles (CELLINK, NZ5221005001 and NZ5251005001). To optimize the printability of materials in the bath, 3-layer cylindrical grid constructs (d=10 mm) were 3D printed.
TeloCol and PhotoCol were transferred to 3 cc cartridges (CELLINK, CSC010300502) using
Alginate Lyophilizate (CELLINK, VLB000000201)
provides a medium viscosity alginate solution sta- ble at room temperature and ionically crosslink- able with CaCl₂. Lyophilized alginate was reconsti- tuted to 2% (w/v) using Reconstitution Agent M (CELLINK, IK200000050). For cell printing, algi- nate solution was mixed with cell suspension in cell medium with a volume ratio of 9:1. For more details, access the FRESH Bioprinting Protocol: Alginate.
RHEOLOGY
Rheological measurements of LifeSupport and collagen biomaterials were performed using an oscillatory shear mode on a rotational Discovery HR-10 Rheometer (TA Instruments) with an aluminum 20mm parallel plate setup with a 0.5mm gap. Temperature ramps were evaluated from5°C to 37°C for collagen materials and from 10°C to 37°C for LifeSupport bath, with a ramp rate of 1°C/min, constant shear strain of 0.5%, and frequency of 1 Hz for all tests. The final stiffness of both collagen materials was measured under constant shear strain and frequency of 0.5% and 1 Hz, respectively, after loading the samples into a 5°C plate and instantaneously increasing the temperature to 37°C for thermal crosslinking. For PhotoCol, an additional step was introduced by exposing the sample to photocrosslinking for 30seconds, using an external LED lamp with near-UV light wavelength (405 nm).
OPTIMIZATION OF FRESH BIOPRINTING
PROCESS
FRESH bioprinting with LifeSupport involves dif- ferent steps shown in Figure 1. The process starts with adding a suspension medium (water, PBS, crosslinking solution, etc.) to the gelatin powder (Figure 1A). The solution is mixed and centrifuged to form the LifeSupport bath (Figure 1B), which is used to fill up a well plate (Figure 1C) or Petri dish. After printing (Figure 1D), the well plate is placed into an incubator at 37°C for 30 minutes for LifeSupport melting (Figure 1E) and the release of printed construct (Figure 1F).
To optimize FRESH bioprinting, one must select an appropriate needle size and adjust printing parameters such as pressure and speed. In our study, the printing optimization was performed using both 22 G and 25 G 1-inch length needles (CELLINK, NZ5221005001 and NZ5251005001). To optimize the printability of materials in the bath, 3-layer cylindrical grid constructs (d=10 mm) were 3D printed.
TeloCol and PhotoCol were transferred to 3 cc cartridges (CELLINK, CSC010300502) using

Figure 1. FRESH bioprinting process:
A) suspension medium addition to the gelatin powder;
B) ready-to-use gelatin based support bath;
C) well plate filledwith LifeSupport;
D) printingof a biomaterial insidethe bath;
E) LifeSupport melting at 37°C;
F) release ofa printed construct.
A) suspension medium addition to the gelatin powder;
B) ready-to-use gelatin based support bath;
C) well plate filledwith LifeSupport;
D) printingof a biomaterial insidethe bath;
E) LifeSupport melting at 37°C;
F) release ofa printed construct.
APPLICATION NOTE
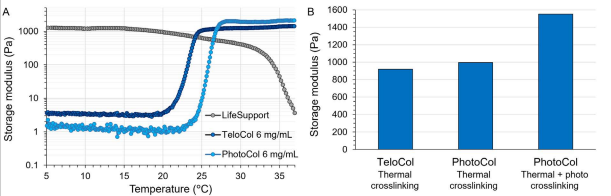
Figure 2. Rheologicalevaluationofthe support bath andcollagenbiomaterials.
A) Materialstability within5°Cto37°C range.
B) Final stiffness ofTeloColandPhotoColafter crosslinking.
a positive displacement pipette for viscous materials. The alginate solution was transferred to 3 cc cartridges using female/female Luer locks (CELLINK, OH000000010). The cartridge was then connected to the selected tip needle (1-inch length) and mounted onto the Temperature- controlled Printhead (CELLINK,000000020346).
Biomaterials were printed using the BIO X bioprinter (CELLINK, D16110020717) with print bed and printhead temperatures set at 10°C and 5°C, respectively, for TeloCol and PhotoCol. Ambient conditions were used for alginate printing. After printing, the well plate was incubated for 30minutes at 37°C (5% CO₂ and 95% relative humidity) for thermal crosslinking of collagen materials and the melting of LifeSupport bath. After thermal crosslinking, PhotoCol samples were exposed to photocrosslinking at 405 nm light for 30 seconds. To avoid premature self-assembly of both collagen biomaterials, consumables and printhead used in the printing were pre-chilled in the freezer. After melting of LifeSupport, alginate constructs may substantially decrease in size due to the shrinking phenomenon caused by prolonged crosslinking in CaCl₂ (typically lasting 5 to 10 minutes).

Figure 2. Rheologicalevaluationofthe support bath andcollagenbiomaterials.
A) Materialstability within5°Cto37°C range.
B) Final stiffness ofTeloColandPhotoColafter crosslinking.
a positive displacement pipette for viscous materials. The alginate solution was transferred to 3 cc cartridges using female/female Luer locks (CELLINK, OH000000010). The cartridge was then connected to the selected tip needle (1-inch length) and mounted onto the Temperature- controlled Printhead (CELLINK,000000020346).
Biomaterials were printed using the BIO X bioprinter (CELLINK, D16110020717) with print bed and printhead temperatures set at 10°C and 5°C, respectively, for TeloCol and PhotoCol. Ambient conditions were used for alginate printing. After printing, the well plate was incubated for 30minutes at 37°C (5% CO₂ and 95% relative humidity) for thermal crosslinking of collagen materials and the melting of LifeSupport bath. After thermal crosslinking, PhotoCol samples were exposed to photocrosslinking at 405 nm light for 30 seconds. To avoid premature self-assembly of both collagen biomaterials, consumables and printhead used in the printing were pre-chilled in the freezer. After melting of LifeSupport, alginate constructs may substantially decrease in size due to the shrinking phenomenon caused by prolonged crosslinking in CaCl₂ (typically lasting 5 to 10 minutes).
CELL PREPARATION
Human mesenchymal stem cells (MSCs) were used for evaluating the influence of the FRESH bioprinting process on cell viability. Cells were expanded in 2D T175 flasks before bioprinting. Throughout the culture, Dulbecco’s modified eagle medium (DMEM) with high glucose (Gibco, 11965092) supplemented with 10% fetal bovine serum (FBS, Gibco, 10270-106) and Antibiotic- Antimycotic (Gibco, 15240096) was used. Before bioprinting, cells in passage 28 were detached from the flask and a cell suspension was prepared with cell viability of 95%.
FRESH 3D BIOPRINTING OF MSCS
The BIO Xwas prepared by cooling theTemperature- controlled Printhead and print bed according to previously described settings. Biomaterials were mixed with the cell suspension for a final concentration of 2 million MSCs/mL. In particular, the cells were added to TeloCol and PhotoCol using a pipette and mixed within the Eppendorf, while the addition of cells to alginate 2% (w/v) solution was done using two 3 mL syringes connected with a Luer lock. The homogeneous solutions were transferred into cartridges and capped with a 22 G 1-inch needle that was chosen as optimal for printing with cells. The following conditions were used for the BIO X bioprinting setup: STL file with cylindrical grid of 5 mm diameter and 1 mm height with one perimeter, infill = 30%, printing speed = 3.5 mm/s, pressure of 10 kPa for the TeloCol and PhotoCol, and 20 kPa for the alginate. The constructs were bioprinted into an untreated 24-well plate (VWR, 7342779) and placed in the incubator at 37°C for 30 minutes to thermally crosslink the constructs and melt the LifeSupport bath. The melted LifeSupport was carefully removed by replacing it with cell culture medium to avoid damaging the printed constructs. Cell culture media were replenished three times a week during the 14-day culturing period.
CELL ANALYSIS
On days 1, 7 and 14 after bioprinting, the viability of the MSCs was assessed using calcein AM (Invitrogen eBioscience, 15560597) and propidium iodide (Sigma Aldrich, 81845-25mg). Cell viability was determined by fluorescent imaging of the constructs with green and red channels. Images were analyzed using ImageJ software to merge live and dead channel images. For constructs, DAPI stain (Thermo Fisher Scientific, 62248) was added on day 14. The protocol for Live and Dead Assay can be found on CELLINK’s website.
APPLICATION NOTE
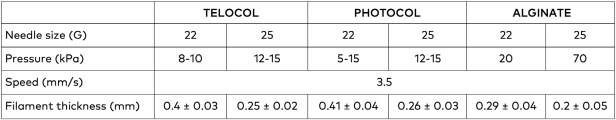
Table1. Filamentthicknessobtained usingoptimized printing parameters for FRESH bioprinting.
Results and discussion
FLOW PROPERTIES AND CROSSLINKING
Rheological tests were performed to check the stability of the temperature-sensitive LifeSupport and collagen biomaterials and ensure satisfactory temperature control during printing. LifeSupport is stable up to 15°C, showing a slight decrease in storage modulus above this temperature. TeloCol and PhotoCol both showed rapid thermal crosslinking above ≈18°C and ≈23°C, respectively (Figure 2A), indicating a need to keep them cold during the printing process to avoid premature collagen network formation and nozzle clogging.
Additionally, the final stiffness of collagen biomaterials was assessed (Figure 2B). TeloCol and PhotoCol showed similar storage modulus values of 920 kPa and 996 kPa, respectively, after thermal self-assembly at 37°C. However, PhotoCol constructs became much stiffer (ie, 1551 kPa) when they were additionally exposed to photocrosslinking after the melting of the LifeSupport bath.
FRESH BIOPRINTING PROCESS
All biomaterials considered for this study were successfully FRESH bioprinted with the extrusion- based BIO X 3D bioprinter. TeloCol printing, however, was challenging because of the need to keep the printhead at 5°C to avoid premature self-assembly. Optimized printing parameters for the three biomaterials and their achieved filament thickness are summarized in Table 1. Fixing printing parameters, the filament thickness for TeloCol and PhotoCol is 0.4 mm and 0.25 mm for a 22 G and 25 G needle, respectively. An excellent result given that the theoretical minimum resolution of a filament is defined by the nozzle diameter. Regarding the alginate printing, the filament thickness is 0.29 mm and 0.2 mm for 22 G and 25 G needles, respectively, demonstrating the possibility to print very thin features, especially with the 25 G needle. Since the relatively high pressure of 70 kPa is not recommended for printing with cells, a 22 G 1-inch needle was chosen for further bioprinting experiments with cells.
Finally, to show how FRESH helps print complex structures, a hollow artery model with a bifurcation (Figure 3A) was 3D printed and perfused. This

Figure 3. Complex structure of hollow artery printed with FRESH method.
A) Hollow artery CAD model. B) Printedconstruct. C) Perfusion ofthe artery.
A) Hollow artery CAD model. B) Printedconstruct. C) Perfusion ofthe artery.
APPLICATION NOTE
model was chosen because it is impossible to print without a support bath or a sacrificial ink. Utilizing FRESH printing, this hollow artery with a bifurcation was printed (Figure 3B) successfully. The construct maintained its structure, and was also perfusable demonstrating the integrity of the print (Figure 3C)
model was chosen because it is impossible to print without a support bath or a sacrificial ink. Utilizing FRESH printing, this hollow artery with a bifurcation was printed (Figure 3B) successfully. The construct maintained its structure, and was also perfusable demonstrating the integrity of the print (Figure 3C)
CELL VIABILITY AND STRETCHING
At day 1 (Figures 4A), MSCs were homogeneously distributed in the printed constructs, indicating proper mixing of biomaterials with cells. Cell viability in TeloCol and PhotoCol constructs was better than in alginate, which can be attributed to the higher printing pressure used for the more viscous alginate bioink. These results show that
FRESH bioprinting could negatively affect the viability of cells in viscous bioinks because of the stress imposed on cells from needle length and prolonged time without cell medium during printing. On day 7 (Figure 4B), there were many MSCs stretching in all directions in TeloCol and PhotoCol constructs. While cells in the alginate constructs were not able to stretch and remained round, as expected for cell-neutral alginate. Day 14 images (Figures 4C) highlight an increased number of stretched cells through the collagen based constructs. There was however, an increased number of dead cells also observed at the center of the constructs. This increase could be attributed to a lack of oxygen and nutrients reaching these cells.

Figure 4. Representative images ofthe bioprinted MSCsstainedwith calceinAM (live cells) and
propidium iodide (dead cells) atA) day 1,B) day7, and C) day14ofculture. Magnification 4x.
APPLICATION NOTE
Conclusion
In this study, we demonstrated the potential of FRESH method using LifeSupport for printing soft and liquid-like collagen and alginate biomaterials using extrusion-based 3D bioprinting. In particular, the proposed method enables printing complex structures without the need for sacrificial support inks or ink modifiers to increase mechanical stability, keeping the biological requirement of the biomaterials.
References
1. Adamiak K, Sionkowska A. Current methods of collagen cross-linking: Review. International Journal of Biological Macromolecules. 2020; 161: 550–560. DOI:10.1016/j.ijbiomac.2020.06.075.
Conclusion
In this study, we demonstrated the potential of FRESH method using LifeSupport for printing soft and liquid-like collagen and alginate biomaterials using extrusion-based 3D bioprinting. In particular, the proposed method enables printing complex structures without the need for sacrificial support inks or ink modifiers to increase mechanical stability, keeping the biological requirement of the biomaterials.
References
1. Adamiak K, Sionkowska A. Current methods of collagen cross-linking: Review. International Journal of Biological Macromolecules. 2020; 161: 550–560. DOI:10.1016/j.ijbiomac.2020.06.075.
2. Adib AA, Sheikhi A, Shahhosseini M, et al. Direct-write 3D printing and characterization of a GelMA-based biomaterial for intracorporeal tissue engineering. Biofabrication. 2020; 12(4): 045006. DOI:10.1088/1758-5090/ab97a1.
3. Hazur J, Detsch R, Karakaya E, et al. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre crosslinking technique. Biofabrication. 2020; 12(4): 045004. DOI:10.1088/1758-5090/ab98e5.
4. Heid S, Boccaccini AR. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomaterialia. 2020; 113: 1 –22. DOI:10.1016/j.actbio.2020.06.040.
5. Hinton TJ, Jallerat Q, Palchesko NR, et al. Science Advances. 2015; 1(9): e1500758. DOI:10.1126/ sciadv.1500758.
6. Jeon O, Lee Y, Hinton T, Feinberg A, Alsberg E. Cryopreserved cell laden alginate microgel bioink for 3D bioprinting of living tissues. Materials Today Chemistry. 2019; 12: 61 –70. DOI:10.1016/j. mtchem.2018.11.009.
7. Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019; 365: 482–487. DOI:10.1126/science.aav90.
8. Shiwarski DJ, Hudson AR, Tashman JW, Feinberg AW. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioengineering. 2021; 5(1): 010904. DOI:10.1063/5.0032777.
9. Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Advanced Materials. 2011; 23(24): H178– H183. DOI:10.1002/adma.201004625.
3. Hazur J, Detsch R, Karakaya E, et al. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre crosslinking technique. Biofabrication. 2020; 12(4): 045004. DOI:10.1088/1758-5090/ab98e5.
4. Heid S, Boccaccini AR. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomaterialia. 2020; 113: 1 –22. DOI:10.1016/j.actbio.2020.06.040.
5. Hinton TJ, Jallerat Q, Palchesko NR, et al. Science Advances. 2015; 1(9): e1500758. DOI:10.1126/ sciadv.1500758.
6. Jeon O, Lee Y, Hinton T, Feinberg A, Alsberg E. Cryopreserved cell laden alginate microgel bioink for 3D bioprinting of living tissues. Materials Today Chemistry. 2019; 12: 61 –70. DOI:10.1016/j. mtchem.2018.11.009.
7. Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019; 365: 482–487. DOI:10.1126/science.aav90.
8. Shiwarski DJ, Hudson AR, Tashman JW, Feinberg AW. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioengineering. 2021; 5(1): 010904. DOI:10.1063/5.0032777.
9. Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Advanced Materials. 2011; 23(24): H178– H183. DOI:10.1002/adma.201004625.
Copyright(C) 1998-2024 生物器材網 電話:021-64166852;13621656896 E-mail:info@bio-equip.com